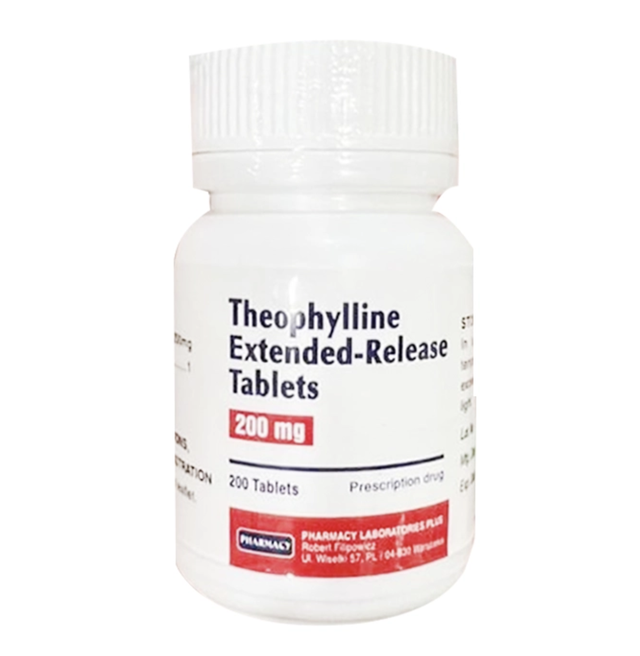
A photo shows Theophylline Extended Release Tablets 200mg, marketed as Theophylin 200mg.
The drug in question, Theophylline Extended Release Tablets 200mg—marketed as Theophylin 200mg—was found to contain only 6.3 percent of the active ingredient stated on its label, according to the Hanoi Drug, Cosmetic, Food Quality Control Center.
The sample was collected from An An Pharmacy in Hanoi’s Ha Dong District.
The batch carries lot number 21127, with the manufacturing date of February 26, 2022, and the expiration date of February 26, 2026.
Although the label lists Pharmacy Laboratories Plus as the manufacturer, it lacks registration or import license information.
The DAV warned that the medication, which is used to treat asthma, may pose serious health risks to patients if it fails to deliver the intended therapeutic effects.
The agency has instructed local health departments to trace the product’s origin, verify its authenticity, and work with police and market surveillance authorities to inspect relevant facilities.
It also called for the increased testing and sampling of drugs at high risk of being counterfeit or substandard, with a special focus on Hanoi.
As part of the investigation, the DAV requested that Hanoi’s health department urgently report findings to the national steering committee on anti-smuggling, trade fraud, and counterfeit goods.
The department is also expected to coordinate inspections of An An Pharmacy and take legal action if violations are found.
The administration urged the public and drug suppliers to avoid purchasing or distributing the affected product and encouraged anyone suspecting counterfeit drugs to report them to the authorities.


Max: 1500 characters
There are no comments yet. Be the first to comment.