
A sample of counterfeit medicine recently detected by Vietnam’s drug regulator is seen in this handout photo. Drug Administration of Vietnam/Handout via Tuoi Tre
The Drug Administration of Vietnam (DAV) said on Friday that it received a report from the Hanoi Drug, Cosmetic and Food Quality Control Center confirming that a batch of DIAMICRON® MR 60mg tablets contained only 42.5mg of gliclazide—just 70.8 percent of the amount stated on the label.
The tablets, from lot number 23F603, are set to expire in April 2026.
Gliclazide is used to treat type 2 diabetes, and the batch in question failed to meet Vietnam’s pharmacopeia standards, the DAV stated.
The medication was being sold at Duc Anh Pharmacy in Hanoi’s Dong Da District.
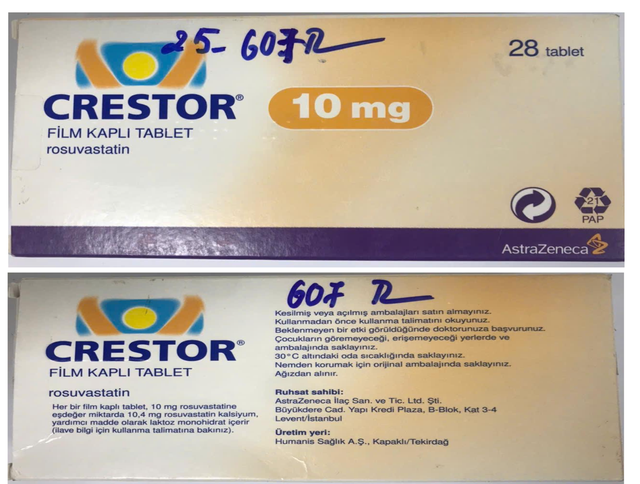
A box of Crestor 10mg (rosuvastatin), lot number A24236004, expiring in July 2027, identified as counterfeit. Drug Administration of Vietnam/Handout via Tuoi Tre
During the same inspection, six other drugs were found to lack valid import licenses or verifiable manufacturer information.
These include: Oseltamivir, lot M1164B01, which expired in March 2023; Crestor 20mg (rosuvastatin), lot A23237030, which expires in April 2026; Janumet 50/1000mg (sitagliptin/metformin), lot 24497505A, which expires in July 2026; Plavix (clopidogrel), lot ELB04027, which expires in May 2027; NEXIUM® 40mg (esomeprazole), lot 23H420, which expires in September 2027; and Crestor 10mg, lot A24236004, which expires in July 2027.
The DAV has instructed the Hanoi Department of Health to coordinate with police and the National Steering Committee on Anti-Smuggling, Trade Fraud and Counterfeit Goods to inspect the pharmacy and trace the origin of these products.
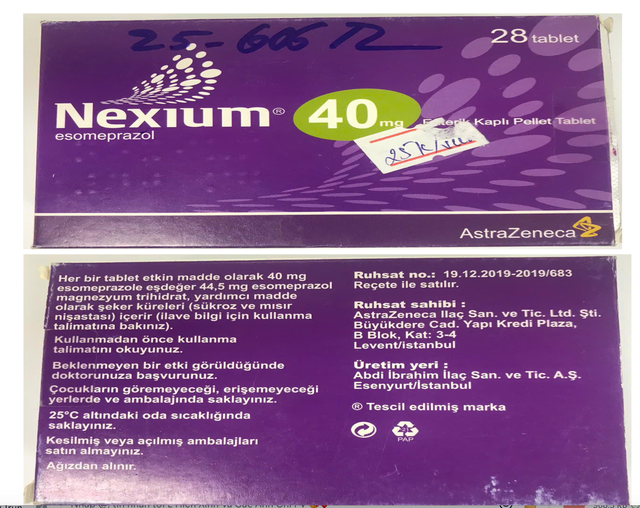
A box of NEXIUM® 40mg (esomeprazole), lot number 23H420, expiring in September 2027, identified as counterfeit. Drug Administration of Vietnam/Handout via Tuoi Tre
Health departments nationwide were urged to alert hospitals, clinics, and the public about the findings and to caution against using the listed products.
“Unregistered or poor-quality drugs pose a risk to public health,” the DAV warned, advising consumers to buy medicines only from licensed pharmacies.
An official report on the investigation is expected by June 2.


Max: 1500 characters
There are no comments yet. Be the first to comment.