Vietnam withdraws anemia drug Femancia over quality violations
The Drug Administration of Vietnam on Thursday revoked the marketing authorization of the anemia drug Femancia, citing repeated quality violations, the Ministry of Health said.
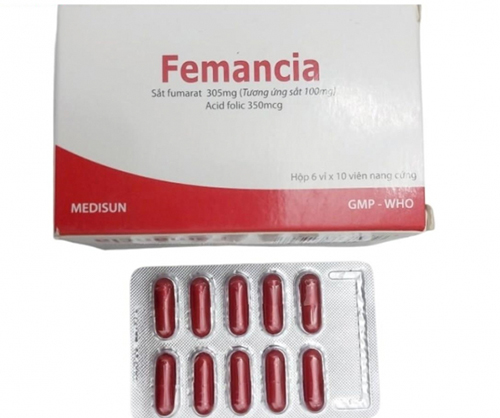
Femancia, a drug used to treat iron-deficiency anemia, is seen in an undated handout photo.
Femancia, produced and registered by MeDiSun Pharmaceutical JSC, was removed from the list of approved pharmaceuticals after two batches were found to violate quality standards at Level 2 severity, according to the ministry.
Under Vietnam’s pharmaceutical law, a drug’s marketing license must be revoked if two or more batches are subject to mandatory recalls for Level 2 violations within a 60-month period.
Femancia, registered under license number VD-27929-17, is a drug used to treat and prevent iron-deficiency anemia.
The drug was manufactured at MeDiSun’s facility in Hoa Loi Ward, Ho Chi Minh City, which was part of old Ben Cat Town, former Binh Duong Province before a recent administrative merger, the ministry said.
A Level 2 quality violation, as defined by the health ministry, indicates there is evidence the drug may be ineffective or pose potential safety risks to users, but not to the extent of causing serious harm or endangering life.
With the revocation, Femancia can no longer be produced or distributed in Vietnam.
MeDiSun is required to recall all distributed products and comply with legal obligations under pharmaceutical regulations.
The Drug Administration has instructed the Ho Chi Minh City Department of Health to monitor MeDiSun’s recall efforts and assess whether the recall has been effectively implemented and if any products remain in circulation that may pose a risk to public health.
Provincial and municipal health departments nationwide have been directed to notify pharmacies and healthcare providers of the recall, publish the withdrawal notice on official websites, and ensure enforcement through inspections and penalties where necessary.
In March 2025, the Drug Administration ordered a recall of two batches of Femancia capsules, which contain 100mg of elemental iron and 350mcg of folic acid, due to failures in potency and dissolution testing, classified as Level 2 violations.
Bao Anh - Duong Lieu / Tuoi Tre News
Link nội dung: https://news.tuoitre.vn/vietnam-withdraws-anemia-drug-femancia-over-quality-violations-103250718163609988.htm